1. For Homework #3, you used the expression E = n²h²/(8ma²) to dete...
Question
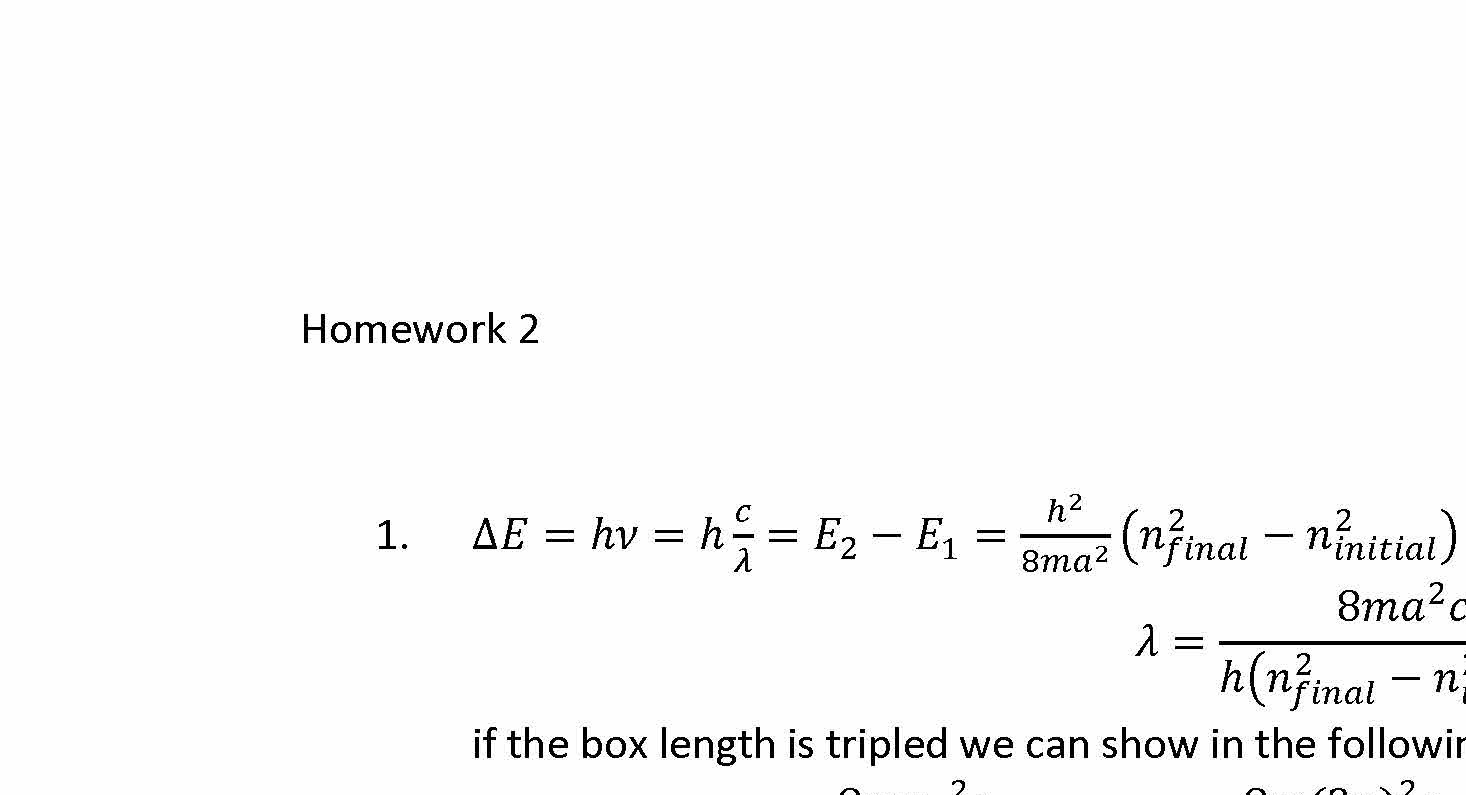
1. For Homework #3, you used the expression E = n²h²/(8ma²) to determine the energies of a particle in a box, and from this we calculated the wavelength of light needed to promote electron-sized particles from n=1 to n=2 levels in some nm-sized boxes. Derive a general expression that allows you to quickly calculate the wavelengths for any ni-->n2 transition (where ni and nz can be any integers), for any particle of mass m, and any box size a.
When the length of the box is tripled, what happens to the wavelength of a given transition such as n=2 to n=3? What happens to the wavelength if the mass of the particle is doubled?
2. Sketch contour maps for the following orbitals:
3py
3dxz
5dz2
When the length of the box is tripled, what happens to the wavelength of a given transition such as n=2 to n=3? What happens to the wavelength if the mass of the particle is doubled?
2. Sketch contour maps for the following orbitals:
3py
3dxz
5dz2
Solution Preview
These solutions may offer step-by-step problem-solving explanations or good writing examples that include modern styles of formatting and construction of bibliographies out of text citations and references.
Students may use these solutions for personal skill-building and practice.
Unethical use is strictly forbidden.
This is only a preview of the solution.
Please use the purchase button to see the entire solution.
Please use the purchase button to see the entire solution.
By purchasing this solution you'll be able to access the following files:
Solution.pdf
Solution.jpg
Solution1.jpg
Purchase Solution
$13.00







View Available
Chemistry Tutors 641 tutors matched
Ionut
(ionut)
Master of Computer Science
Hi! MSc Applied Informatics & Computer Science Engineer. Practical experience in many CS & IT branches.Research work & homework
5/5 (6,804+ sessions)
1 hour avg response
$15-$50 hourly rate
Pranay
(math1983)
Doctor of Philosophy (PhD)
Ph.D. in mathematics and working as an Assistant Professor in University. I can provide help in mathematics, statistics and allied areas.
4.6/5 (6,688+ sessions)
1 hour avg response
$40-$50 hourly rate
Leo
(Leo)
Doctor of Philosophy (PhD)
Hi!
I have been a professor in New York and taught in a math department and in an applied math department.
4.9/5 (6,435+ sessions)
2 hours avg response