THE REACTION OF A FOOD DYE WITH BLEACH Introduction In the H2SO3/...
Question
THE REACTION OF A FOOD DYE WITH BLEACH
Introduction
In the H2SO3/KIO3 experiment performed last week, you learned how to determine the order of a reaction with respect to a reactant by measuring the rate of a reaction as a function of the concentration of that reactant.
In this experiment you will take another approach to finding reaction order. If the concentration of a reactant "A" is measured as a function of time, the order can be determined by graphing the results. For example, you can plot [A] VS. time, In [A] vs. time, or 1/[A] vs. time. The linear plot indicates the order with respect to A.
This is because the integrated rate laws that you derived in CHEM 132 can be applied.
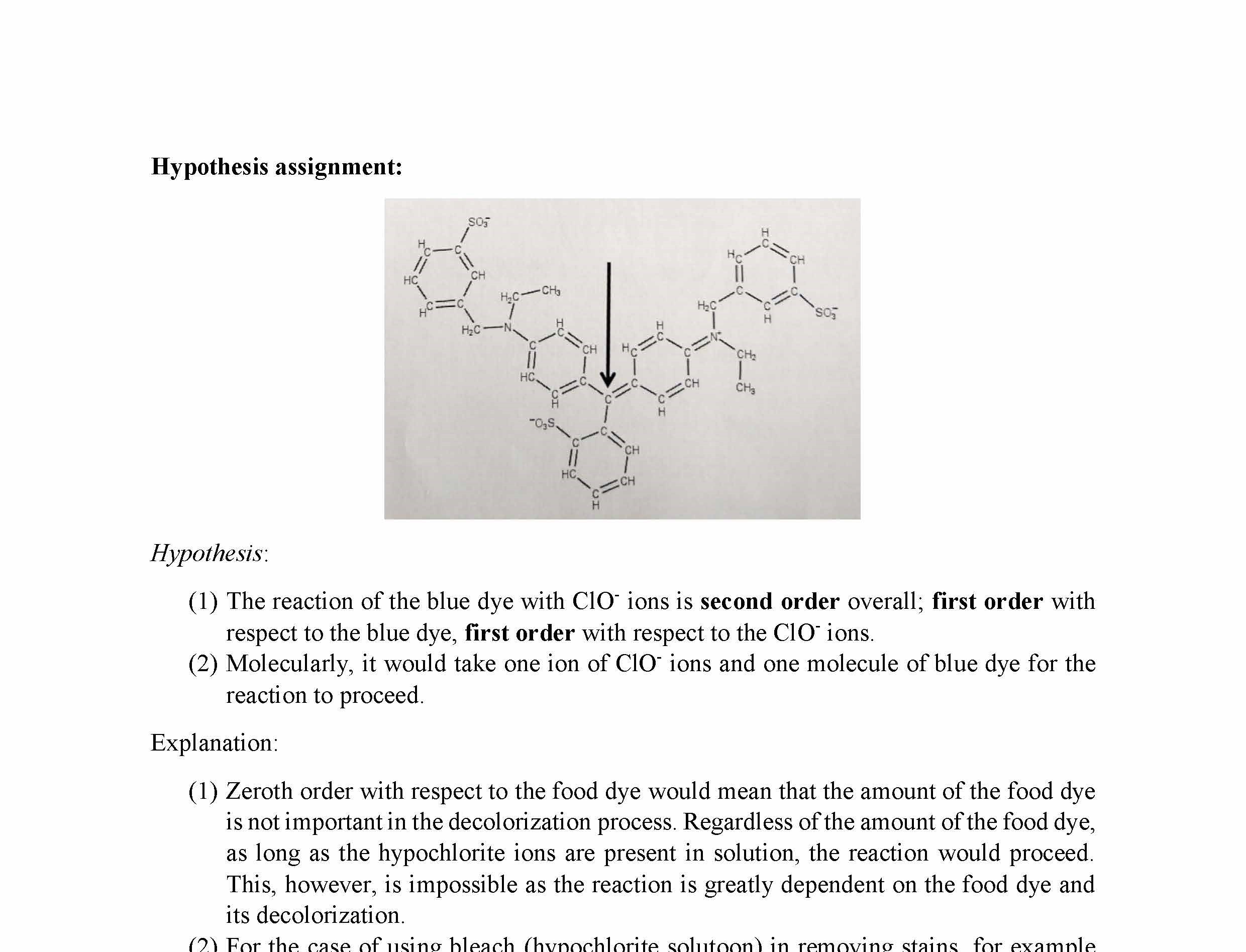
The reaction you will study in this experiment is between blue food coloring (FD&C Blue #1) and NaOCI, the bleaching agent in commercial Clorox.:
FD&C Blue #1 + NaOCI ------> products
Because of the bleaching action, the blue color disappears over time to give a colorless solution. Since the Abs of the blue dye obeys Beer's law (Abs = bC), Abs is directly proportional to the concentration (C) of the blue dye. Therefore, the progress of the reaction can be followed using a spectrophotometer to measure the absorbance (Abs) of the blue dye as a function of time.
The rate law for the above reaction is:
Rate = k[NaOCI]¹ [Blue #1]˟
Note that the reaction is first order with respect to the bleach. Your overall goal in this experiment is to determine X (the order with respect to the blue dye) and then to calculate the value of k. If this reaction is carried out with a high [NaOCI], it is said to be conducted under "pseudo-first-order conditions", and the rate law simplifies to:
Rate = Kbserved [Blue #1]˟ (where K observed =k[NaOCI]¹)
The value of K observed can be found from the slope of the appropriate plot of concentration data vs. time.
Then k can be calculated from the known concentration of NaOCI in Clorox.
Hypothesis
Before coming to the laboratory you and your partner must write a hypothesis for this experiment. To do so, answer the following questions: What do you predict to be the value of X (the order with respect to the blue dye)? On what basis do you make this prediction? How will you use the concentration vs. time data to prove your predicted order?
Procedure
Only the bare essentials of the procedure are given below. Before coming to the laboratory you and your partner must write a detailed procedure on how you are going to do the experiment.
It is recommended that you use the Clorox without dilution and that you prepare a stock solution of the FD&C Blue #1 by adding about 6 drops of the dye to 400 mL of distilled water.
You will have the following special equipment available: Spectronic 20 spectrophotometers, burets (for measuring out the desired amounts of Clorox), and stopwatches.
Part A: Determine a max of the FD&C Blue #1
You will need to use your stock solution to determine the wavelength of maximum absorbance (Amax) of the blue dye. This is the wavelength that you will use in Part C of this experiment.
Consider: How will you calculate the molar concentration of the blue dye in your stock solution based on this data? (Hint: For the blue dye, the molar absorptivity ) is 1.38 X 105 M' cm"¹.)
Part B: Determine the amounts of Clorox and blue dye solution to use in the rate study The purpose of this part is to find the right amounts of the Clorox and blue dye solution to combine when you do the rate study with the spectrophotometer in Part C. If the concentrations are too high, then the reaction will be too fast and may even be complete before you get your mixture into the Spectronic 20 cuvet!
If the concentrations are too low, then it may take too long to see significant changes in the absorbance.
You will determine the right amounts to use by visual observations. It is recommended that you use 1.00 mL of Clorox (buret) with various volumes (graduated cylinder) of your blue dye stock solution. For example, you might begin by combining 1.00 mL of Clorox with 10.0 mL of the blue dye solution and then observe how long it takes for the blue solution to become colorless. Your goal should be to find a combination that causes the reaction to be complete (i.e., become colorless) in about 15 minutes.
Part C: Make absorbance VS. time measurements
Now you should be ready to measure the absorbance as a function of time at the Amax value found in Part A, using the appropriate combination of blue dye and Clorox discovered in Part B. (Don't forget to blank the Spectronic 20 with water.) Mix together the desired combination of the two reactants, quickly transfer some of the mixture to a cuvet, and immediately begin recording absorbance as a function of time. It is recommended that you record the absorbance each minute (or 30 seconds if you are so inclined) for a total reaction time of 11-12 minutes.
Graphing and Calculations
Although you are not required to complete the graphing and calculations during the lab period, you and your partner are expected to come to the lab with a plan on how you are going to plot your Part C data and how you are going to do the calculations to find X and the value of k in the rate law for this reaction. You must know how to do the following calculations:
(1) How will you determine the order (x) from your graphs?
(2) How will you determine Kobserved from your graphs?
(3) What is the molarity (M) of the NaOCI (in the cuvet) in Part C? First you will need to calculate the original M of NaOCI in commercial Clorox. (Hint: The Clorox bottle is labeled as 8.25% (w/w) NaOCI, and you can assume that its density is 1.00 g/mL.)
The M of NaOCI in the cuvet depends on how much the NaOCI is diluted when you combine 1.00 mL of the Clorox with the volume of blue dye you selected. This is just a dilution problem.
(4) How will you calculate the value of k from the above information?
Introduction
In the H2SO3/KIO3 experiment performed last week, you learned how to determine the order of a reaction with respect to a reactant by measuring the rate of a reaction as a function of the concentration of that reactant.
In this experiment you will take another approach to finding reaction order. If the concentration of a reactant "A" is measured as a function of time, the order can be determined by graphing the results. For example, you can plot [A] VS. time, In [A] vs. time, or 1/[A] vs. time. The linear plot indicates the order with respect to A.
This is because the integrated rate laws that you derived in CHEM 132 can be applied.
The reaction you will study in this experiment is between blue food coloring (FD&C Blue #1) and NaOCI, the bleaching agent in commercial Clorox.:
FD&C Blue #1 + NaOCI ------> products
Because of the bleaching action, the blue color disappears over time to give a colorless solution. Since the Abs of the blue dye obeys Beer's law (Abs = bC), Abs is directly proportional to the concentration (C) of the blue dye. Therefore, the progress of the reaction can be followed using a spectrophotometer to measure the absorbance (Abs) of the blue dye as a function of time.
The rate law for the above reaction is:
Rate = k[NaOCI]¹ [Blue #1]˟
Note that the reaction is first order with respect to the bleach. Your overall goal in this experiment is to determine X (the order with respect to the blue dye) and then to calculate the value of k. If this reaction is carried out with a high [NaOCI], it is said to be conducted under "pseudo-first-order conditions", and the rate law simplifies to:
Rate = Kbserved [Blue #1]˟ (where K observed =k[NaOCI]¹)
The value of K observed can be found from the slope of the appropriate plot of concentration data vs. time.
Then k can be calculated from the known concentration of NaOCI in Clorox.
Hypothesis
Before coming to the laboratory you and your partner must write a hypothesis for this experiment. To do so, answer the following questions: What do you predict to be the value of X (the order with respect to the blue dye)? On what basis do you make this prediction? How will you use the concentration vs. time data to prove your predicted order?
Procedure
Only the bare essentials of the procedure are given below. Before coming to the laboratory you and your partner must write a detailed procedure on how you are going to do the experiment.
It is recommended that you use the Clorox without dilution and that you prepare a stock solution of the FD&C Blue #1 by adding about 6 drops of the dye to 400 mL of distilled water.
You will have the following special equipment available: Spectronic 20 spectrophotometers, burets (for measuring out the desired amounts of Clorox), and stopwatches.
Part A: Determine a max of the FD&C Blue #1
You will need to use your stock solution to determine the wavelength of maximum absorbance (Amax) of the blue dye. This is the wavelength that you will use in Part C of this experiment.
Consider: How will you calculate the molar concentration of the blue dye in your stock solution based on this data? (Hint: For the blue dye, the molar absorptivity ) is 1.38 X 105 M' cm"¹.)
Part B: Determine the amounts of Clorox and blue dye solution to use in the rate study The purpose of this part is to find the right amounts of the Clorox and blue dye solution to combine when you do the rate study with the spectrophotometer in Part C. If the concentrations are too high, then the reaction will be too fast and may even be complete before you get your mixture into the Spectronic 20 cuvet!
If the concentrations are too low, then it may take too long to see significant changes in the absorbance.
You will determine the right amounts to use by visual observations. It is recommended that you use 1.00 mL of Clorox (buret) with various volumes (graduated cylinder) of your blue dye stock solution. For example, you might begin by combining 1.00 mL of Clorox with 10.0 mL of the blue dye solution and then observe how long it takes for the blue solution to become colorless. Your goal should be to find a combination that causes the reaction to be complete (i.e., become colorless) in about 15 minutes.
Part C: Make absorbance VS. time measurements
Now you should be ready to measure the absorbance as a function of time at the Amax value found in Part A, using the appropriate combination of blue dye and Clorox discovered in Part B. (Don't forget to blank the Spectronic 20 with water.) Mix together the desired combination of the two reactants, quickly transfer some of the mixture to a cuvet, and immediately begin recording absorbance as a function of time. It is recommended that you record the absorbance each minute (or 30 seconds if you are so inclined) for a total reaction time of 11-12 minutes.
Graphing and Calculations
Although you are not required to complete the graphing and calculations during the lab period, you and your partner are expected to come to the lab with a plan on how you are going to plot your Part C data and how you are going to do the calculations to find X and the value of k in the rate law for this reaction. You must know how to do the following calculations:
(1) How will you determine the order (x) from your graphs?
(2) How will you determine Kobserved from your graphs?
(3) What is the molarity (M) of the NaOCI (in the cuvet) in Part C? First you will need to calculate the original M of NaOCI in commercial Clorox. (Hint: The Clorox bottle is labeled as 8.25% (w/w) NaOCI, and you can assume that its density is 1.00 g/mL.)
The M of NaOCI in the cuvet depends on how much the NaOCI is diluted when you combine 1.00 mL of the Clorox with the volume of blue dye you selected. This is just a dilution problem.
(4) How will you calculate the value of k from the above information?
Solution Preview
These solutions may offer step-by-step problem-solving explanations or good writing examples that include modern styles of formatting and construction of bibliographies out of text citations and references.
Students may use these solutions for personal skill-building and practice.
Unethical use is strictly forbidden.

This is only a preview of the solution.
Please use the purchase button to see the entire solution.
Please use the purchase button to see the entire solution.
By purchasing this solution you'll be able to access the following files:
Solution.pdf
Purchase Solution
$60.00







View Available
Chemistry Tutors 641 tutors matched
Ionut
(ionut)
Master of Computer Science
Hi! MSc Applied Informatics & Computer Science Engineer. Practical experience in many CS & IT branches.Research work & homework
5/5 (6,804+ sessions)
1 hour avg response
$15-$50 hourly rate
Pranay
(math1983)
Doctor of Philosophy (PhD)
Ph.D. in mathematics and working as an Assistant Professor in University. I can provide help in mathematics, statistics and allied areas.
4.6/5 (6,688+ sessions)
1 hour avg response
$40-$50 hourly rate
Leo
(Leo)
Doctor of Philosophy (PhD)
Hi!
I have been a professor in New York and taught in a math department and in an applied math department.
4.9/5 (6,435+ sessions)
2 hours avg response